Bronsted Lowry Base Is Defined as Which of the Following
The BrønstedLowry theory is an acidbase reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The electron pair of the base B forms a new bond to H c.

Classify The Following As Acid Or Base According To Bronsted Lowry Concept I Ch3coo Ii H3o Iii So4 2 Iv Hcl
The acid H-A loses a proton leaving an electron pair on atom A b.
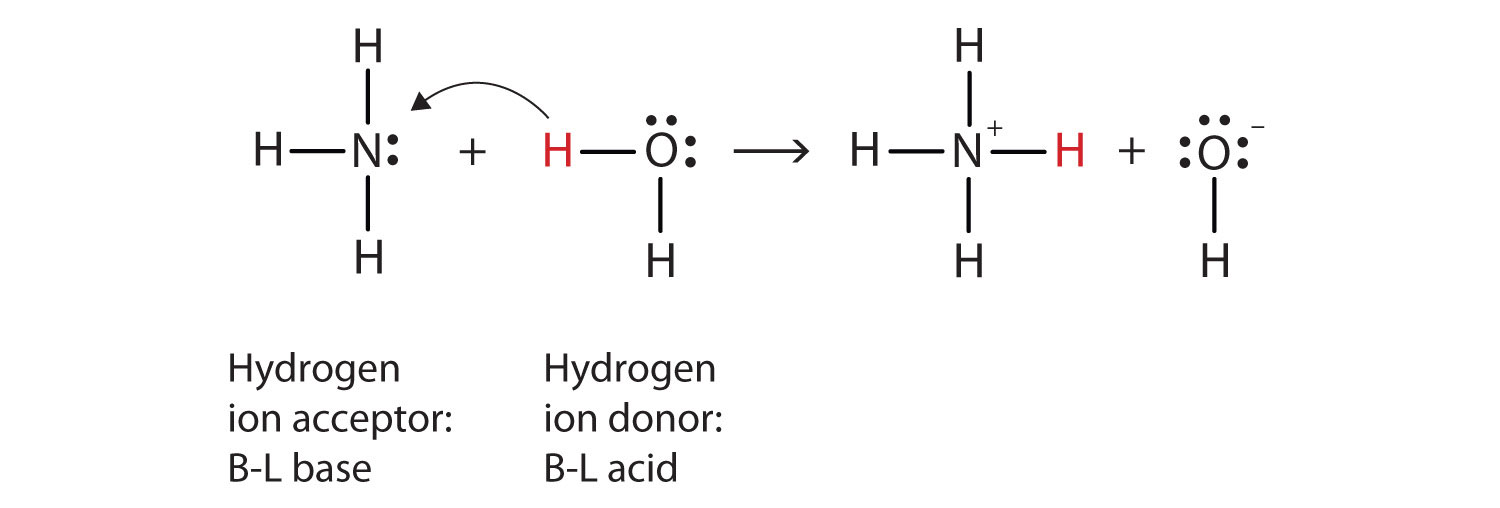
. A Brønsted-Lowry acid is any species that can donate a proton and a base is any species that can accept a proton. Which of the following statements correctly describe the steps involved in any general reaction bw a Bronsted-Lowry acid and base see the diagram a. N H 3 H N H 4.
The Brønsted-Lowry theory describes acid-base interactions in terms of proton transfer between chemical species. Hence they are Bronsted-Lowry acids in aqueous solution. As understood achievement does not recommend that you have fabulous.
Bronsted - Lowry base is a chemical species that is capable of accepting a proton which requires a lone pair of electrons to bond the hydrogen ion. The acids becomes its conjugated base. Download Free Bronsted Lowry Acid And Base Guided Answer Bronsted Lowry Acid And Base Guided Answer Yeah reviewing a book bronsted lowry acid and base guided answer could accumulate your near connections listings.
Strong Acid Bronsted-Lowry loses H with difficulty which means that its conjugate base is a strong base. Which of the following describes what type of solution this is. D produces OH in solution.
This is just one of the solutions for you to be successful. This theory is a. Classify the reactants in the following reactions as an acid or base according to the Bronsted-Lowry Definition.
This is best illustrated in the following equation. Bronsted - Lowry base is a chemical species that is capable of accepting a proton which requires a lone pair of electrons to bond the hydrogen ion. The Arrhenius theory where acids and bases are defined by whether the molecule contains hydrogen and hydroxide ion is too limiting.
A a proton donor. Which of the following formulas is used to determine the pH of a solution. Dissolves in water to yield hydroxide ions.
According to the Bronsted-Lowry definition which of the following is formed when a base dissociates in water. H I H I N H 4 N H 3 H H C O 3 C O 3 2 H H 2 S H S H Bronsted-Lowry bases in aqueous solution can accept a proton H. In a model that precedes the Bronsted-Lowry model a base is defined as something that __________.
B a proton acceptor. PH -logH A solution of wheat flour and water has a H of 1 x 10-8M. Acids ______ and bases ________.
E none of the above 102 In examining the formula for acetic acid HC2H302 the ionizable hydrogen atom s isare. Classify each product as the conjugate acid or base. Which of the following is a characteristic of Lewis bases.
It accepts a proton to become a conjugate acid. The base B loses an electron pair and gains a positive charge d. In this newer system Brønsted-Lowry acids were defined as any molecule or ion that is capable of donating a hydrogen cation proton H whereas a Brønsted-Lowry base is a species with the ability to gain or.
To solve this problem Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923 both independently proposed an alternative definition of acids and bases. According to the Bronsted-Lowry definition NH2 will react with water accoriding to the following chemical equation. Weak Acid Bronsted-Lowry donate protons accept protons.
10 Why is Arrhenius theory still used. 14 Which of these is. 13 What is the difference between the Arrhenius and the brønsted Lowry definitions of a base.
The main effect of the Brønsted-Lowry definition is to identify the proton H transfer occurring in the acid-base reaction. Terms in this set 20. In other words it is a species that has a lone electron pair available to bond to H.
Hence it is Bronsted-Lowry base in aqueous solution. It is named after the Danish chemist Johannes Nicolaus Brønsted and the English chemist Thomas Martin Lowry defines an acid as a proton donor and a base as a proton acceptor. H3O According to the Brønsted-Lowry definition of acids and bases which of the following results when an acid loses an H ion.
The fundamental concept of this theory is that when an acid and a base react with each other the acid forms its conjugate base and the base forms its conjugate acid by exchange of a proton. N H 3 can accept a proton. In 1923 a broader definition of acids and bases was independently proposed by Danish chemist Johannes Brønsted 18791947 and English chemist Thomas Lowry 18741936.
Because it accepts a hydrogen ion. 9 What is Arrhenius definition of acid class 10. Bronsted Lowry conceptA more general acid-base theory the Brønsted-Lowry theory.
Brønsted-Lowry theory of acids and bases. A the H on the left B one of the Hs on the right-side. C produces H in solution.
A Brønsted-Lowry base is a molecule or ion that accepts a hydrogen ion in a reaction. Consider the following chemical equation. When it donates its proton the acid becomes its conjugate base.
In contrast a Bronsted-Lowry base accepts hydrogen ions. A Brønsted-Lowry acid is a molecule or ion that donates a hydrogen ion in a reaction. 12 Which of the following is an example of Arrhenius acid quizlet.
11 What happens when an Arrhenius acid reacts with an Arrhenius base. Which of the following substances is defined as one that donates H ions. 101 The Bronsted-Lowry definition of a base is.
A more general look at the theory is an acid as a proton donor and a base as a proton acceptor. Loses H easily which means that its conjugate base is weak. A Bronsted-Lowry base is a chemical species capable of accepting a proton.
A hydrogen ion is commonly. The conjugate acid of a Bronsted-Lowry base forms once it accepts a proton. A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction.
After a Bronsted-Lowry acid donates a proton it forms its conjugate base.
Acids And Bases The Bronsted Lowry Definition

Ch 18 Chem 211 Niu Flashcards Quizlet

Bronsted Lowry Definition Of Acid Base Acid Base Equilibria Mcat Content

5 1 Acid Base Definitions Conjugate Acid Base Pairs General Chemistry For Gee Gees
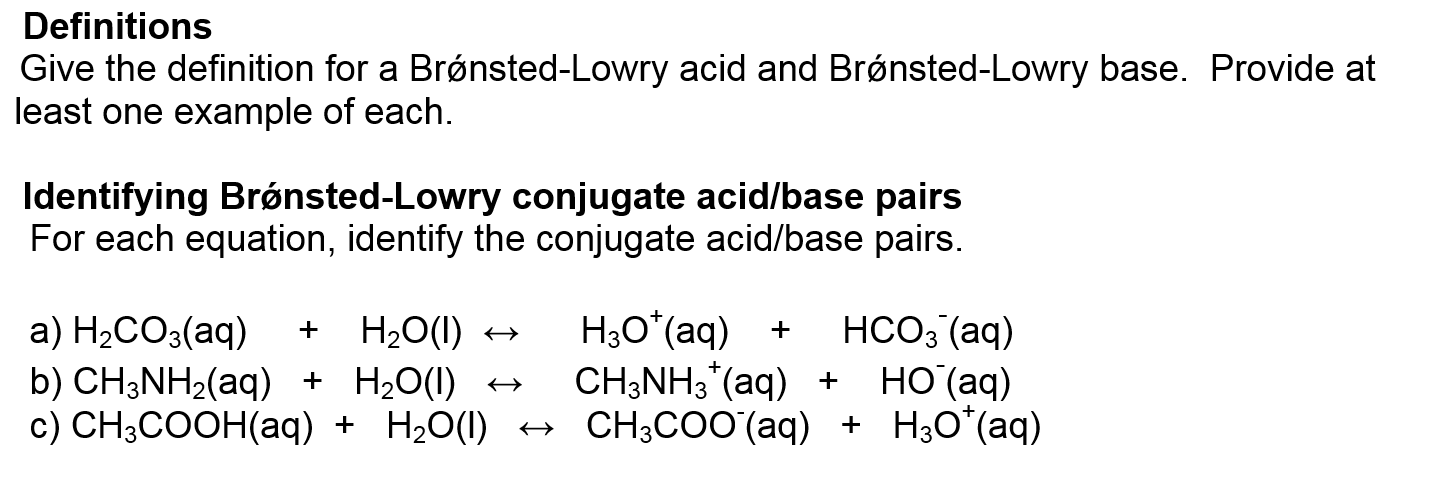
Solved Give The Definition For A Bronsted Lowry Acid And Chegg Com

Bronsted Lowry Acids And Bases Mcc Organic Chemistry

Bronsted Lowry Acids And Bases Chemistry For Majors
Acids And Bases The Bronsted Lowry Definition
What Are Bronsted Acids And Bases Example

Bronsted Lowry Acids And Bases Chemistry For Majors

Bronsted Lowry Acids And Bases Chemistry 2e
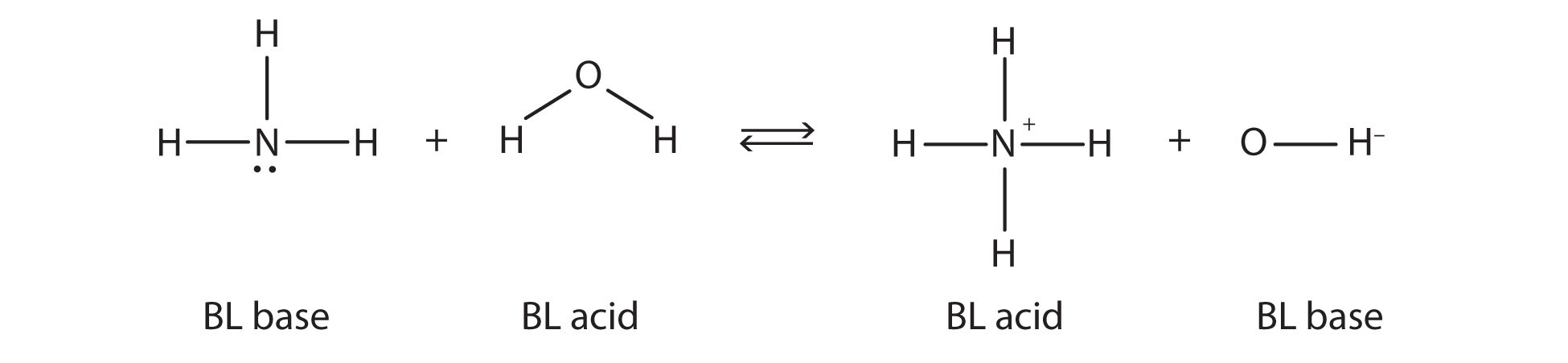
7 2 Bronsted Lowry Acids And Bases Chemistry Libretexts

Mcat General Chemistry Chapter 10 Acids And Bases Flashcards Quizlet

Acids And Bases Chapter 16 Johannes N Bronsted Thomas M Lowry Ppt Video Online Download
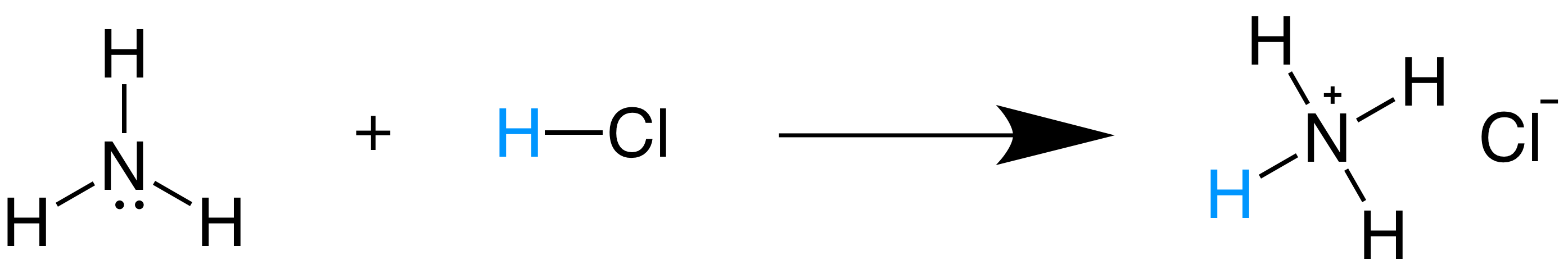
Bronsted Lowry Acids And Bases Article Khan Academy
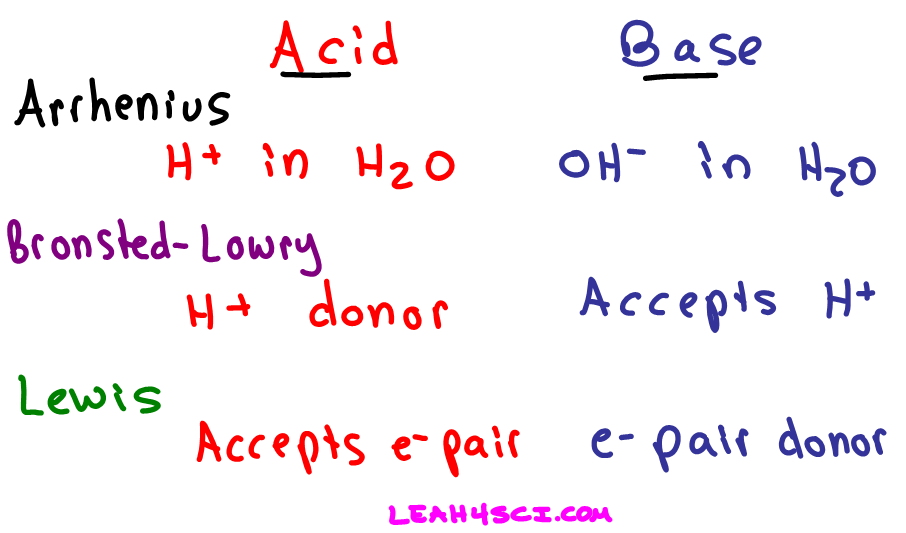
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry

Acids And Bases Flashcards Quizlet

Bronsted Lowry Base In Inorganic Chemistry Bartleby

10 4 Bronsted Lowry Definition Of Acids And Bases Chemistry Libretexts
Comments
Post a Comment